

WHITE PAPER
Your Pharmacy’s Guide to DSCSA 2025:
Exemptions, Insights and Strategies


The United States Food and Drug Administration just announced further updates and exemptions for new Drug Supply Chain Security Act (DSCSA) requirements for pharmacies.
These updates follow the FDA already postponing the enforcement of new DSCSA compliance requirements.
The updates vary based on each trade partner’s status, role and size. They also include new requirements for pharmacy compliance, adding more complexity to the already complicated DSCSA regulations. There are still substantial penalties for non-compliance.
Delays in enforcement don’t mean time to relax; they warrant additional preparation and proactive measures. This white paper will summarize the latest DSCSA requirements, advise pharmacies on their next steps, and share best practices for purchasing and vendor compliance. The paper will also cover mandated data, strategies and solutions for maintaining pharmacy compliance.
DSCSA Compliance: Basics and Risks
DSCSA encompasses rules for tracking prescription drugs across the entire supply chain. The FDA enacted this regulation in 2013 to ensure patient safety and secure a safe chain of custody. It requires pharmacies, manufacturers and wholesalers to comply with and consistently follow regulation updates. Non-compliance entails audits, financial penalties, suspension or legal action.
For pharmacy purchasing, the latest DSCSA updates require pharmacies to record and store package-level product-tracing information before receiving any items. That means both transaction information (TI) and transaction statement (TS) data (also known as “T2” data). Pharmacies must receive T2 data using the historical interoperable standard known as EPCIS and store six years of data in an electronic format.
Pharmacies must also update their receiving process to check for suspicious products at the package level. The requirement to quarantine suspect products, report them within 24 hours and respond to audit requests within 48 hours is still in effect. These new DSCSA requirements were set to take effect on November 27, 2024.
New DSCSA updates demand proactive compliance from pharmacies.
Latest DSCSA Updates and Exemptions
On October 9, 2024, to prevent supply chain disruptions and ensure uninterrupted patient access to medications, the FDA announced further exemptions for DSCSA enforcement. Now, trading partners who have made documented efforts or successfully established data connections with immediate partners but still face challenges may be exempt from DSCSA requirements.
The exemption timelines vary by the type and size of the trading partner:
- Dispensers with 25 or fewer full-time licensed employees: until November 27, 2026
- Dispensers with 26 or more full-time licensed employees: until November 27, 2025
- Manufacturers and repackagers: until May 27, 2025
- Wholesale distributors: until August 27, 2025
Pharmacies must determine eligibility for these exemptions based on their licensed employee count as of November 27, 2024. Pharmacies do not need to notify or apply to the FDA for the new exemptions. However, if pharmacies determine that the new exemptions do not apply to them but they are still not ready for the November 27, 2024, enforcement deadline, they can apply to the FDA for a waiver.
Pharmacies should read the official FDA announcement about exemptions and seek the advice of a licensed attorney for any legal questions or concerns.
Suggested Pharmacy Best Practices and Next Steps
While the language above is ambiguous, “documented efforts” might include:
- Setting up global location numbers (GLNs) and sharing them with trading partners or solution providers like SureCost
- Requesting vendors send EPCIS files to solution providers (like SureCost)
- Updating standard operating procedures for how you electronically store or access vendor transaction information (such as EPCIS)
In addition, pharmacies might be able to demonstrate sufficient challenges among trading partners based on reasonable evidence from the industry, such as:

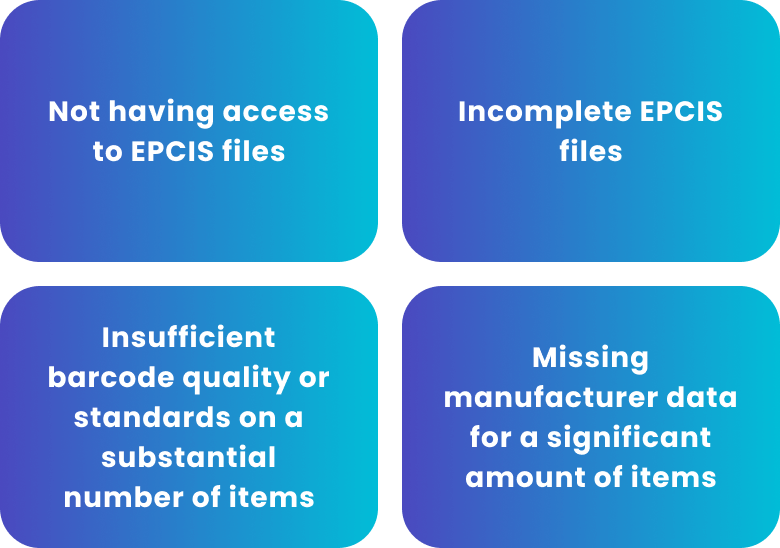
Pharmacies should continue to prepare for the new deadlines based on their current status. That includes acquiring GLNs for all locations and sharing them with all vendors and purchasing partners.
They should continue encouraging trading partners to be EPCIS-compliant and share records as needed. Pharmacies that decide to scan non-DSCSA-exempt items may encounter challenges due to the number of products with barcode issues. Given wholesalers’ extended deadline, these pharmacies may want to delay scanning efforts. At the same time, they should continue to ensure that their staff and standard operating procedures are ready for the new DSCSA requirements.
New DSCSA exemptions ease compliance timelines — pharmacies must assess eligibility.
Four Essentials for Optimal DSCSA Compliance
DSCSA requirements were always complex. However, the regulation is even more complicated with the change to package-level data verification and new technical specifications. Here are four key strategies to ensure pharmacy compliance without complicating operations or stressing your staff.
Unify data
New DSCSA regulations require electronic receipt and storage of data. Technology is no longer a luxury; it’s a necessity for DSCSA compliance. Manual processes are unacceptable. Complex, outdated platforms and spreading data across multiple systems confuse the process. This approach cannot guarantee the level of data organization and transparency needed to comply with the new DSCSA requirements.
Pharmacies need a single source of truth for DSCSA-related data. Since this data ties back to receiving purchased products, managing all data using a unified pharmacy purchasing and inventory management solution is the best option.
Instead of looking at multiple purchasing interfaces for each vendor and combining all DSCSA-relevant data from different sources, one integrated pharmacy purchasing solution can track and record everything from submitting a purchase order to receiving items.
The system instantly logs relevant information as staff scan items at receiving. At the same time, it verifies the pharmacy is getting the right quantity of the correct item for the agreed-upon price. Capturing all data at every procurement stage in real time, the system can also facilitate prompt and accurate reporting about DSCSA, purchasing history, vendor compliance and all aspects of procurement. For example, a single built-in report for “T2” takes away hours and assures accurate data during an audit.
Ensure Accountability of Vendors
There are multiple negative consequences for not keeping up with DSCSA regulations or the tools needed to maintain compliance:
Fines
Damaged vendor relations
Penalties
Loss of patient confidence
Legal sanctions
Stress on staff
Suspension
Failing to spot even one mismatched product identifier could lead to any of these impacts. Yet, with pharmacies receiving multiple products all the time and staff already busy, they need a method to automatically flag anything that doesn’t match the vendor’s data. Pharmacies also need the capability to quarantine items if they suspect tampering or other issues quickly.
The right purchasing management solution ensures pharmacies can meet these needs. It means accountability for vendors supplying the data and for staff collecting and managing these products. For example, technology can align teams on consistent protocols for quarantine and reconciliation. It can also help them purchase strategically by driving to the right items based on vendor compliance or savings. If staff need to make an exception (e.g., buying a different item due to patient preference), the manager can see why. With purchasing data and reports, leadership can also monitor for shrinkage.
Unify data, hold vendors accountable, streamline operations.
Pharmacies Must Be Ready to Quarantine and Respond to Audits
When product identifier information doesn’t match the vendor’s data, pharmacies need a method to flag the issue quickly. Ideally, that’s an automatic process that sends alerts when DSCSA data doesn’t match. From there, a clear view of all flagged items allows them to reconcile each issue. Pharmacies may be able to obtain a new matching record from the vendor. They might need to rescan the product in case of scanning or keying errors.
Pharmacies are also responsible for efficiently responding to audits within the requisite timeframe. That means keeping data for the required six-year timeframe. It also entails furnishing all requisite data within the mandated timeframe in case of an audit. Besides storing the data, pharmacies must be ready to generate reports in response to these requests.
These situations are stressful enough on their own; if the time comes, pharmacies should have a solution to quickly and correctly furnish the right data. These data protocols and security measures should be built into the technology.
Knowledgeable, experienced pharmacy professionals understand the urgency and requirements behind DSCSA compliance. The best pharmacy solutions reflect these insights. With the right team behind these solutions, the solution will meet the latest DSCSA regulations. But their experience in the industry also allows them to design technology that fulfills these functions without burdening pharmacies with complex training or lengthy onboarding.
Maintain Data Between Transfers
Pharmacies with multiple branches or within complex health systems must manage these data and responsibilities at every step. When they move products between locations, they need to track those transfers while keeping the chain of custody intact. That means integration with inventory management as well as pharmacy purchasing.
Fortunately, technology allows pharmacies to see each step of the transfer process, from initiating the request to receiving the item at the destination. At the same time, they can scan DSCSA barcode data for any inbound/outbound transfers across all locations.
If there are any discrepancies, pharmacy staff need the ability to flag non-matching traceable items when receiving inbound transfers while quarantining and then tracking non-compliant traceable transferred items.
Pharmacy purchasing solutions offering mobile capabilities enable teams to scan items from their portable devices quickly. It’s another way to spot discrepancies or suspect products immediately and quickly reconcile issues at the source or initiate required protocols. But it also “untethers” staff from their desk; they go between your desk and the receiving area. Teams go where the work is.
Automate DSCSA alerts, track transfers, and ensure rapid audit response readiness.
The Full Context for Effective DSCSA Compliance
DSCSA Checklist for Data and Pharmacy Responsibilities
Capture mandated information, including package-level data:
Manage T2 data
Track DSCSA barcode data for inbound/outbound transfers:
Verify package-level info from and between:
Satisfy technical and administrative requirements:
Maintain high-quality patient care and business operations:
New DSCSA requirements put an even greater burden on pharmacies regarding purchasing and inventory. While the latest exemptions may seem like a “breather” for some pharmacies, the regulation is too complex and the stakes are too high to sit back. Pharmacies must stay ready by keeping up with the DSCSA regulations and optimizing how they can remain compliant.
A surefire method to consistently stay DSCSA-compliant is automating the capture, verification and storage of DSCSA pharmacy data. Yet, this shouldn’t be a standalone process. Pharmacies should simultaneously optimize purchasing and inventory management with a unified solution based on advanced technology designed by partners who understand their needs.

Download the white paper
Complete this form to get the PDF version of our report.
